Research
The Gebhard Lab is an interdisciplinary scientific research group, which aims to generate a better understanding of sex- and gender-specific disease mechanisms of cardiovascular diseases and critical illness in order to improve the care of patients of all genders.
Mission statement
At the Gebhard Lab, we bring gender equality together with cardiovascular and intensive care medicine through cutting-edge research and state-of-the-art education.
Projects
Sex Differences in Cardiac Function
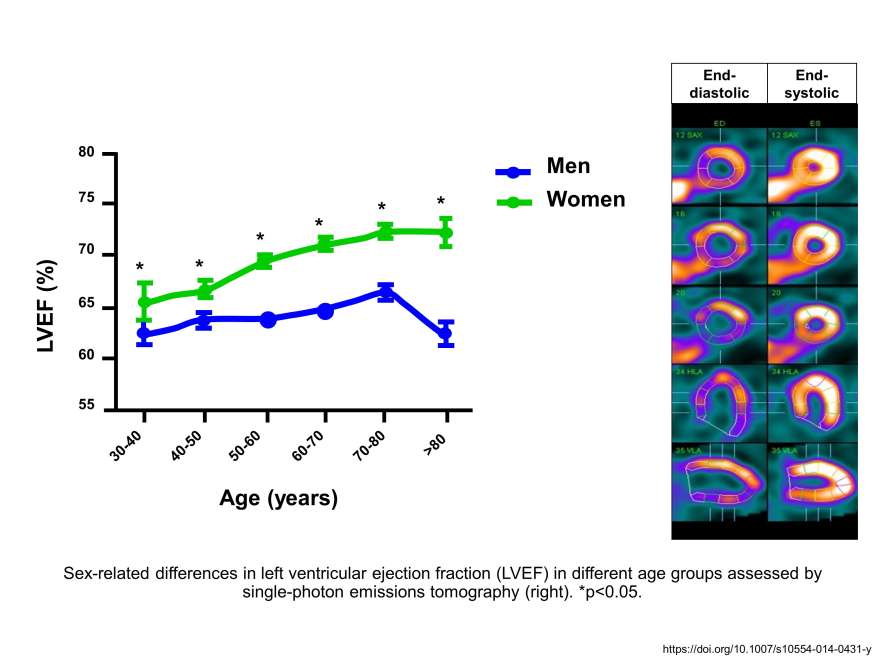
Our recent findings show significant sex- and age-specific differences in baseline left ventricular ejection fraction (LVEF) with a strong age-dependent increase in LVEF in healthy women, but not in men.
Sex and Gender Differences in Critical Illness
Sex and gender inequalities in the provision of intensive care treatment
The Heart-Brain Axis
The female cardiovascular system is more sensitive to stress.
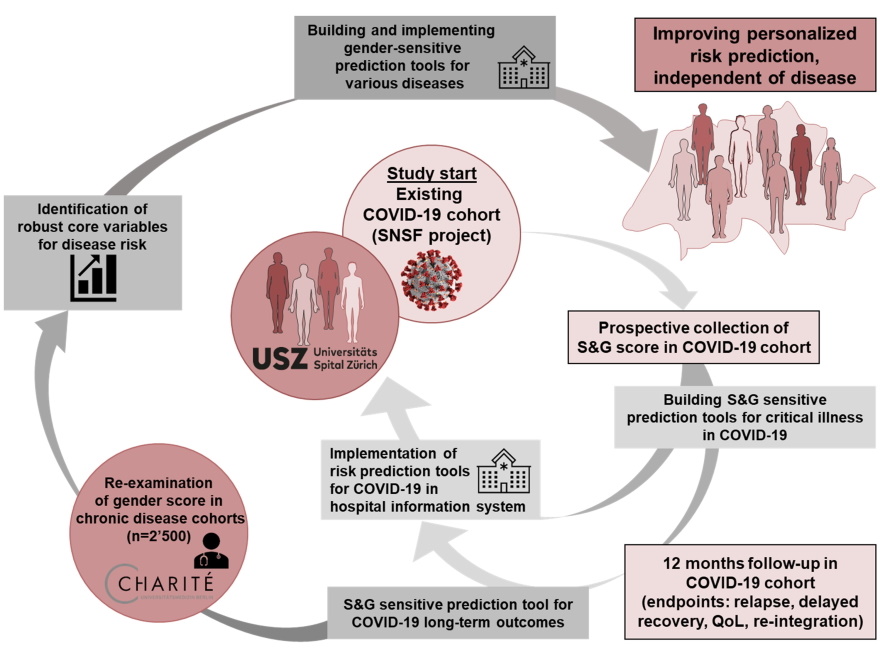
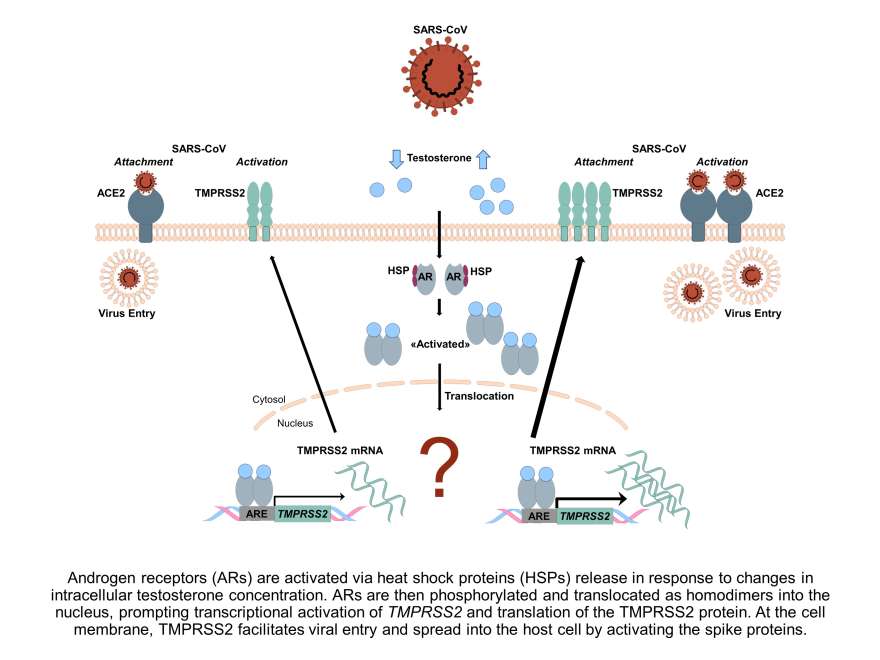
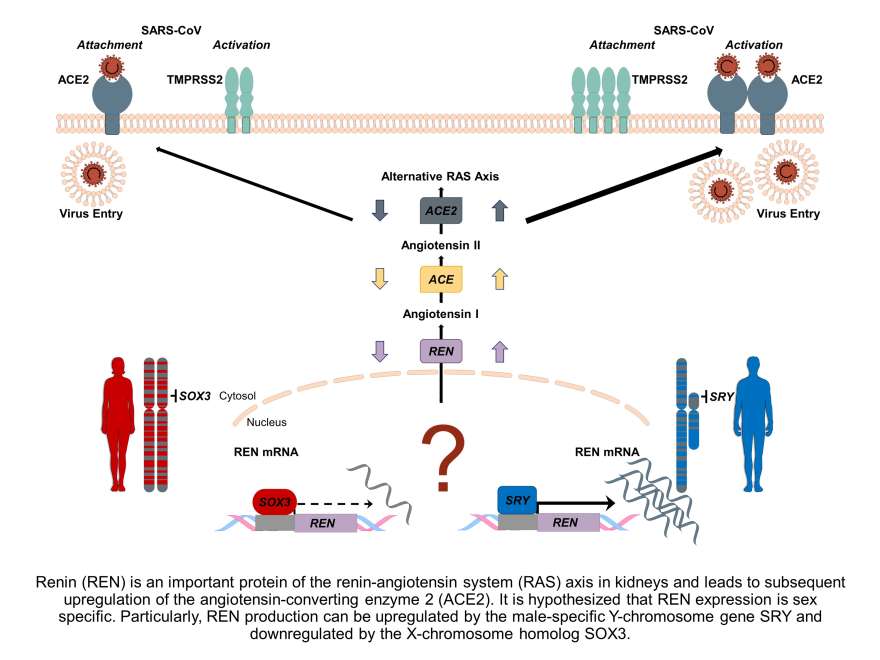
Impact of Sex and Gender on COVID-19 Outcomes
Men are more susceptible to a severe disease course of COVID-19 than women, and worldwide data show that the disease is deadlier in men than in women.
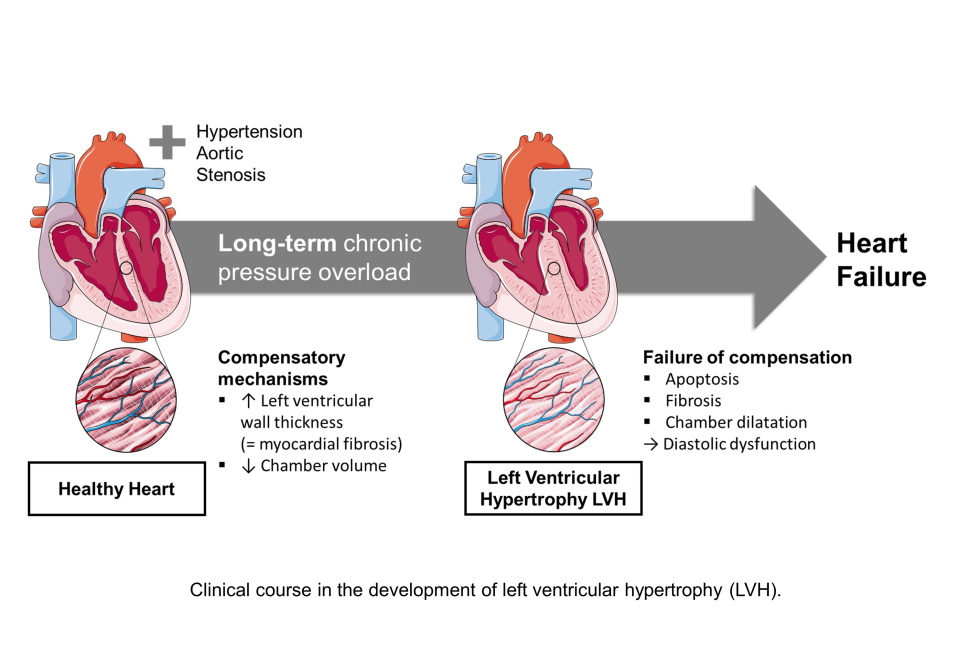
Cardiac Hypertrophy
Left ventricular hypertrophy (LVH) is an example of a heart disease with significant sex-specific differences.
Men’s Health
Life expectancy is shorter in men than in women, and premature mortality is twice as high in men than in women. What are the reasons?
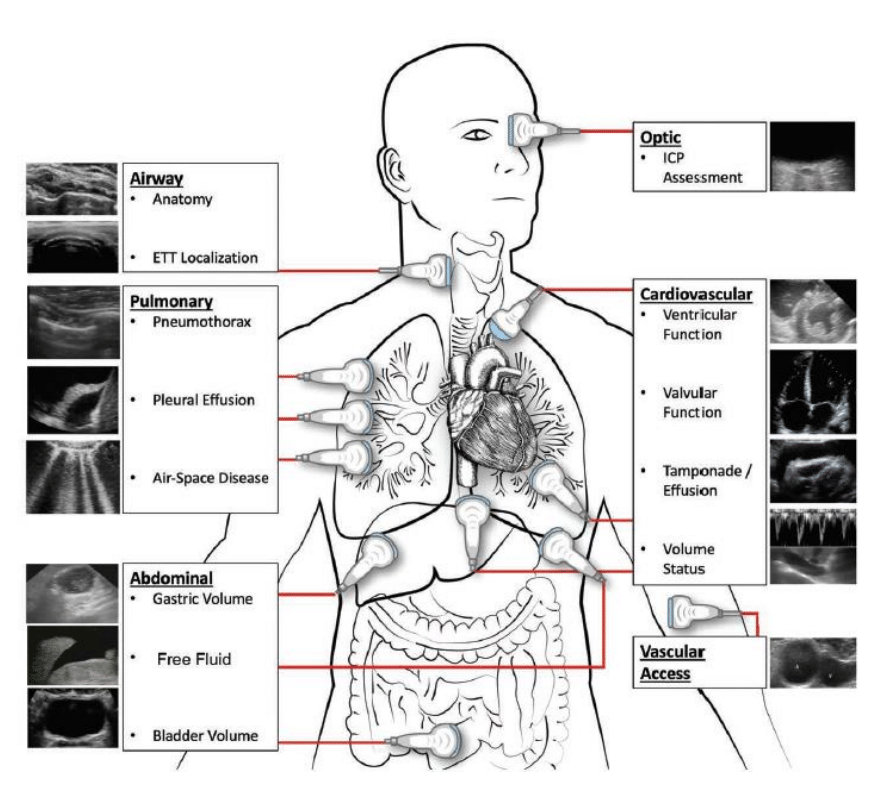
Personalized Medicine In Critically Ill Patients Using Bedside Sonography
Beside sonography is a powerful tool to diagnose and monitor critically ill patients.
Sex and Gender Differences in Myocardial Perfusion
Assessment of sex-specific determinants of myocardial blood flow.
Sex and Gender Differences in Coronary Artery Disease
Coronary artery disease (CAD) differs between women and men in terms of risk factors, clinical presentation, pathophysiology, and prognosis.
Sex, Gender, and the Sympathetic Nervous System
The sympathetic nervous system may play a key role in rendering the female cardiovascular system more susceptible to the detrimental effects of mental stress.
Sex Differences in Cardiac Function
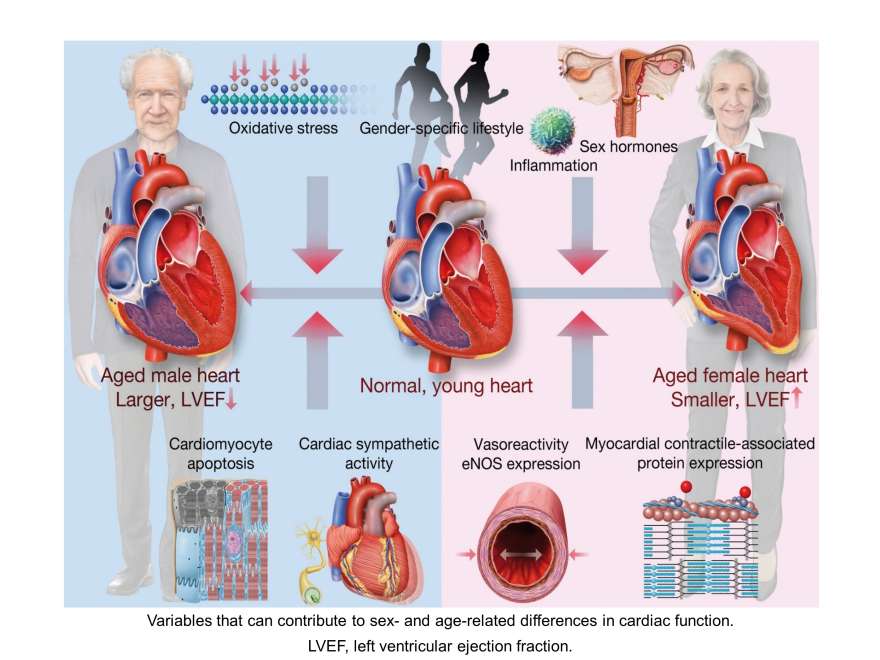
Since left ventricular ejection fraction (LVEF) is routinely used in clinical decision making, it is vital to understand the variables that control cardiac contractility and its vulnerability to injury in postmenopausal women and older men. These insights are critical for the development of personalized age- and gender-based therapies. We therefore assess the impact of (patho)physiological factors contributing to sex- and age-related differences in cardiac function. Specifically, we investigate whether these sex-differences can be attributed to sex hormones and their receptors, differences in genetic predisposition, neurohumoral signalling, and gender-based lifestyle factors. Our studies use murine experimental models and diverse imaging techniques like positron-emissions tomography (PET), echocardiography, and cardiovascular magnetic resonance (cMR) imaging. Our ultimate goal is to improve and personalise gender-based treatments for the aging population.
Clinical Studies
Corpataux et al., Int J Cardiol. 2025
Schütze et al., Int J Cardiol. 2025
Luu et al., J Cardiovasc Magn Reson. 2022
Gebhard et al., Eur Heart J Cardiovasc Imaging. 2020
Gebhard et al., Eur Heart J Cardiovasc Imaging. 2017
Gebhard et al., Int J Cardiovasc Imaging. 2014
Gebhard et al., Echocardiography. 2013
Fiechter et al., MC Med Imaging. 2013
Review Articles
Interviews
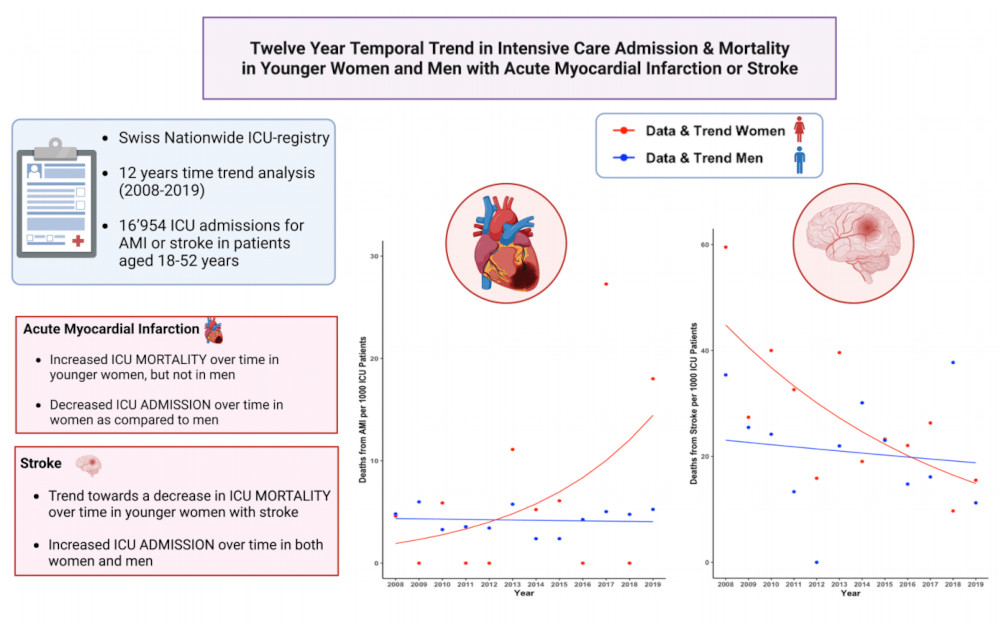
Sex and Gender Differences in Critical Illness
While there is increasing awareness that sex and gender impact presentation, treatment, and disease course of critically ill patients, observational studies consistently report persisting gender disparities in the delivery of intensive care treatment. In fact, we have recently shown that women were less often admitted to intensive care units (ICUs) in Switzerland, despite being equally or more severely ill than their male counterparts. Moreover, given the wide spectrum of diseases seen at the ICU, profound inter-individual variations in epidemiology, pathophysiology and presentation, disease severity, and responses to treatment are present, many of which can be attributed to the distinct impact of sex hormones on the immune and cardiovascular system. This heterogeneity of critically ill patients represents a tremendous challenge for healthcare staff and emphasizes the need to systematically integrate sex and gender considerations into the care of critically ill patients. Therefore, our current research aims to better understand factors contributing to sex and gender differences in critically ill patients with the ultimate goal to overcome current inequalities between male and female ICU patients.
Clinical Studies
Amacher et al., Ann Intensive Care. 2025
Amacher et al., Crit Care. 2025
Zimmermann et al., Crit Care. 2024
Baumann et al., Crit Care. 2023
Arslani et al., Crit Care. 2023
Knobel et al., J Clin Monit Comput. 2023
Todorov et al., Intensive Care Med. 2021
Interviews & Media
SRF || DE
Medinside || DE
Watson || DE
Swissinfo || DE
Primenews || DE
Deutsches Gesundheitsportal || DE
Badische Zeitung || DE
Healthcare-in-europe || DE
SRF2 || DE
Review Articles
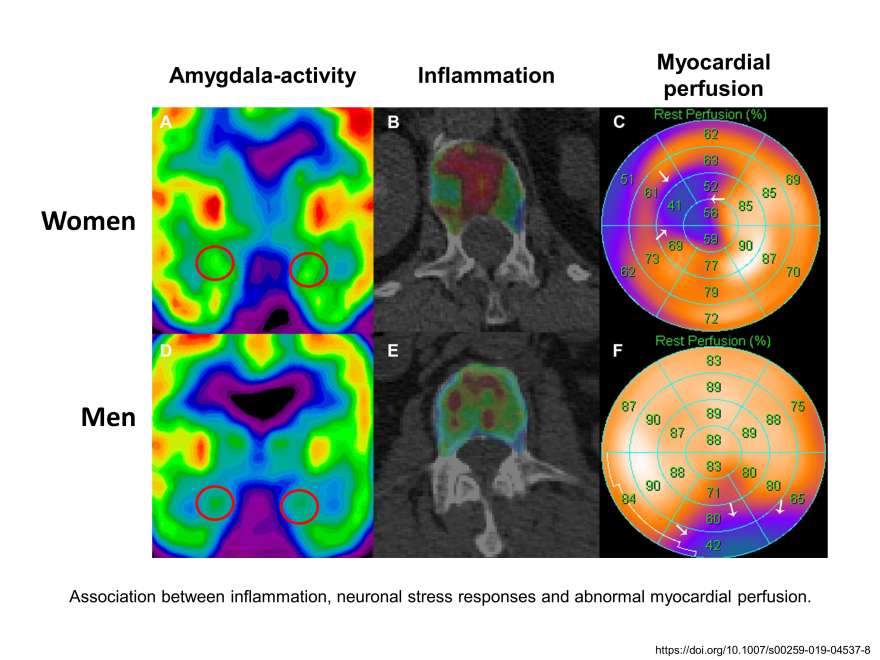
The Heart-Brain Axis
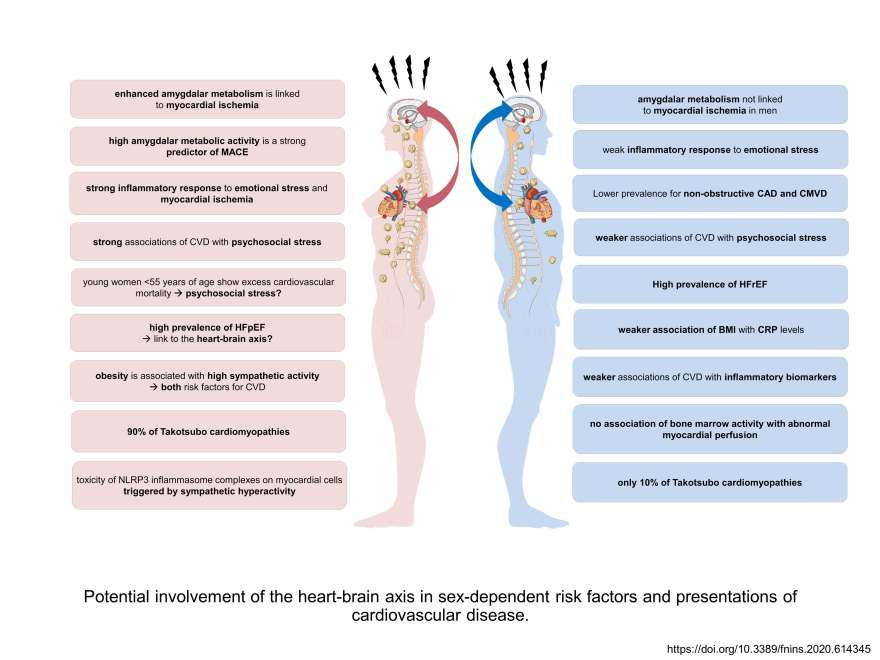
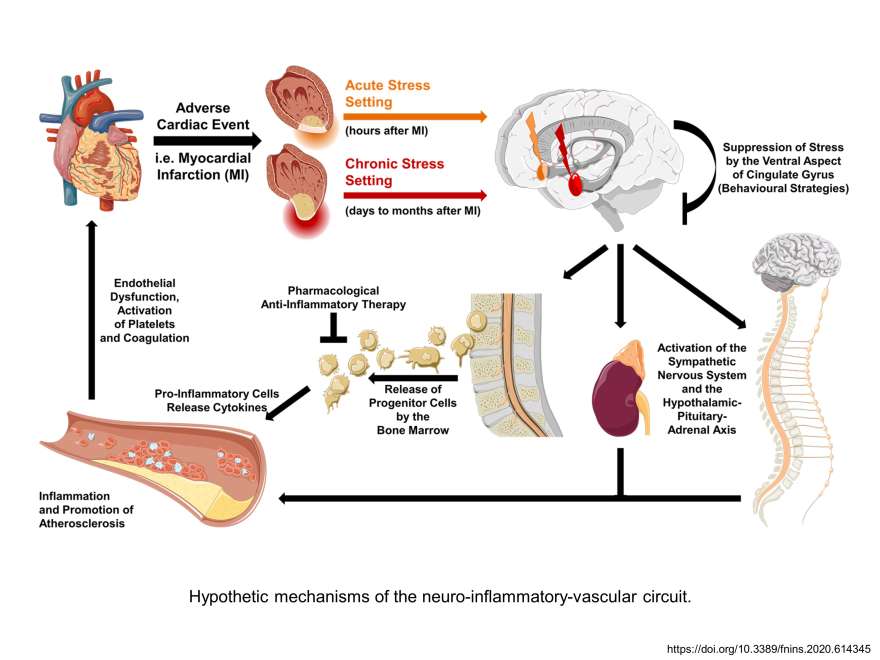
Using the latest imaging techniques, the Gebhard Lab has demonstrated that the amygdala, a part of the brain that controls emotional reactions (the so-called anxiety centre), is highly active in women with cardiac cardiovascular disorders, but not in men. It is hypothesized that increased rates of inflammatory markers in the blood and bone marrow form a link between the stress response in the brain and the heart disease in women. Therefore, stress reduction in women with heart disease should become a vital part of cardiovascular disease management. In a follow-up project, we are now investigating further control mechanisms at the molecular and cellular level that are responsible for the increased stress response in women with heart disease to develop specific and effective cardiovascular treatments.
More Information
Clinical Studies
Mikail et al., Nat Commun. 2025
Albertini et al., Eur Heart J Cardiovasc Imaging. 2025
Civieri et al., JACC Adv. 2024
Rohner et al., J Am Heart Assoc. 2024
Heuer et al., Cerebrovasc Dis. 2024
Mikail et al., Eur Heart J. 2024
Hänsel et al., Eur J Neurol. 2022
Diggelmann et al., J Pers Med. 2021
Haider et al., J Nucl Cardiol. 2021
Fiechter et al., Eur J Nucl Med Mol Imaging. 2020
Fiechter et al., J Am Heart Assoc. 2019
Fiechter et al., Eur Heart J Cardiovasc Imaging. 2019
Review Articles
Tay et al., Theranostics. 2025
Khalil et al., J Nucl Cardiol. 2024
Mikail & Gebhard., Circ Cardiovasc Imaging. 2024
Gebhard et al., Eur Heart J. 2023
Glarner et al., Prim Hosp Care Allg Inn Med. 2022
Rossi et al., Eur Heart J. 2022
Gebhard et al., Front Neurosci. 2020
Liberale et al., Front Neuroendocrinol. 2018
Basic Research Articles
Television Appearances
Lectures
Lecture "Heart-brain Crosstalk in Takotsubo Cardiomyopathy: Does Sex Matter?" at ESC congress, London (2024)
Lecture "Sex differences in heart-brain interactions: a cardiology perspective" at ESC congress, London (2024)
Lecture "Sex Differences in Heart-brain Interaction – the Cardiologist’s Perspective" at EAN congress, Helsinki (2024)
Lecture "Sex und Gender in der Neurologie" at the University Hospital Zurich (2024)
Lecture "The brain-hormone connection: investigating the Influence of sex hormones" at the 3rd International Danube Symposium, Vienna (2023)
Lecture «Heart-Brain Interactions» from the postgraduate CAS study programme in Sex- and Gender-specific Medicine, Switzerland (2022)
Consensus Documents/Guidelines
Impact of Sex and Gender on COVID-19 Outcomes
COVID-19 mortality is highest in aged men and men with pre-existing cardiovascular diseases. The mechanisms underlying these differences are currently unclear. We analyse available clinical and epidemiological data on ageing, co-morbidities and lifestyle in Switzerland to elucidate the role of sex and gender variables in the COVID-19 disease progression. In addition, the underlying mechanisms will be assessed in a murine model of hormone withdrawal. Our work aims to identify both the biological sex- and gender-related predictors for COVID-19 and in order to develop effective antiviral interventions.
More Information
Basic Research Articles
Clinical Studies
Cardiac Hypertrophy
It is an independent risk factor for cardiovascular complications, including heart failure, sudden cardiac death, and stroke. Although women are at a higher risk for left ventricular hypertrophy (LVH)-associated complications, the unique sex-specific determinants remain unknown. We are investigating the role of cardiac fibrosis, inflammation and sympathetic activity in the progression of LVH in women. Given the increasing proportion of women dying from cardiovascular disease and the greater life expectancy of women in the developed world, it is imperative that we develop our knowledge about the role of cardiac hypertrophy in the ageing female heart. This helps to implement novel diagnostic strategies and a timely risk assessment in both men and women with LVH.
Review Articles
Men’s Health
Premature mortality is twice as high in men than in women based on a 2011 European Commission report. Sociocultural (gender) differences between men and women play a major role in this as poor lifestyles and preventable risk factors account for half of premature deaths in men. In fact, men are more likely to eat unhealthy diets, exercise less, consume more often alcohol and drugs than women, smoke or exhibit risky behaviour. They are also more likely than women to neglect pain and disease, resulting in a general underutilisation of health care services and a lower likelihood to engage in routine checks. Our current research focuses on identifying specific risk and prognostic markers of prostate cancer or cardiovascular disease in men as well as on diseases that are underestimated in men, such as osteoporosis.
Personalized Medicine In Critically Ill Patients Using Bedside Sonography
Beside sonography is a powerful tool to diagnose and monitor critically ill patients in the intensive care unit (ICU). Over the last decade, ultrasound assessment has gained increasing importance for the bedside assessment of hemodynamically unstable patients. Besides thorough assessment of the heart to detect cardiac dysfunction, dysfunction of the heart valves, or life-threatening conditions such as cardiac tamponade, bedside ultrasound also comprises assessment of the lungs, abdomen, brain, and vessels. The concept of such multi-organ ultrasound imaging has recently been named as WHole BOody Ultrasound (WHOBUS). Applying the concept of WHOBUS, our research aims to better understand disease pathology in critically ill patients with the ultimate goal to deliver rapid and personalized treatment. Our projects focus particularly on the application of WHOBUS in the diagnosis and treatment monitoring of right heart dysfunction. In addition, we currently investigate strategies to overcome gender inequalities in medical ultrasound education.
Clinical Studies
Denault et al., Br J Anaesth. 2022
Malinovska et al., Can J Anaesth. 2021
Denault et al., Crit Care Explor. 2020
Gebhard et al., J Cardiothorac Vasc Anesth. 2019
Denault et al., Can J Anaesth. 2020
Gebhard et al., J Clin Med. 2021
Malinovska et al., Can J Anaesth. 2021
Reviews
Case reports
Book Chapters
Resuscitative Transesophageal Echocardiography. Felipe Teran, Bret P. Nelson, Robert Arntfield. Chapter 20: Transesophageal Lung Ultrasound. CE Gebhard, EJ Couture, AY Denault. Springer; 1st edition. 2019.
A Practical Approach to Transesophageal Echocardiography. Albert C Perrino. Chapter 18: Critical Care Echocardiography. EJ Couture, CE Gebhard, AY Denault Lippincott Williams&Wilki; 4th edition. 2019.
Conference talks
Conference: «Focused cardiac ultrasound – cardiac and extracardiac parameters», SGUM, Davos; 05/2023
Conference: «Cardiac and extracardiac assessments of RV dysfunction», SMACT, Guadalajara, Mexico; 05/2023
Conference: «TTE- POCUS in shock», WINFOCUS (World Interactive Network Focused On Critical UltraSound; 12/2022.
Lecture: «Gendermedicine- is there a role in surgery and intensive care medicine? », Departments of Surgery, University Hospital Basel; 11/2022.
Sex and Gender Differences in Myocardial Perfusion
While declines in cardiovascular mortality were primarily attributed to the improved survival of male patients, limited data availability on female-specific characteristics of coronary artery disease (CAD) has fueled sex-disparities in cardiovascular outcomes – with alarming consequences for women. Indeed, contemporary imaging modalities for the diagnosis of CAD exhibit higher test accuracy in men than in women, whereas key features complicating CAD assessment in women include: (1) technical artefacts related to breast tissue and a higher heart rate, (2) smaller heart and vessel size, and reduced exercise capacity, (3) and the higher prevalence of non-obstructive CAD in women. Women are more likely to present with ischemia without evidence of epicardial stenosis (INOCA) on conventional testing procedures. Given the remarkable advances in myocardial perfusion imaging technologies, we envision that optimized imaging protocols hold promise to improve contemporary management of CAD in women. Our team has devoted significant efforts to identify sex and gender variables that affect the readouts of myocardial perfusion imaging examinations, with the aim to establish optimized diagnostic procedures that are tailored to the individual needs of women and men.
Basic Research Articles
Clinical Studies
Sex and Gender Differences in Coronary Artery Disease
Coronary artery disease (CAD) differs between women and men in terms of risk factors – with a higher impact of traditional cardiovascular risk factors (CVRFs) in women, despite a lower overall risk burden, clinical presentation – more often ‘atypical’ in women, mechanisms – with lower atherosclerotic plaque burden in women, and outcomes – worse prognosis in women, despite lower CAD burden. In addition, women more frequently display non-traditional CVRFs, such as mental stress and depression.
A deeper understanding of the potential mechanisms and non-traditional risk conditions modulating the course of disease in women and men, such as unrecognized psychosocial factors and sex-specific vascular and neural stress responses is urgently needed. Our research focuses on sex-specific risk factors of CAD such as menopause, vascular inflammation, environmental risk factors, and stress as well as clinical presentation, treatment strategies, and outcomes of women and men presenting with CAD.
Reviews
Regitz-Zagrosek and Gebhard, Nat. Rev. Cardiol. 2022
Mikail et al., Eur J Nucl Med Mol Imaging. 2022
Accompanying editorial: Eur J Nucl Med Mol Imaging. 2022
Haider et al., Eur Heart J. 2020
Clinical Studies
Roa-Díaz et al., Menopause. 2023
Raguindin et al., Clin Endocrinol (Oxf). 2022
Todorov et al., Intensive Care Med. 2021
Bengs et al., Eur J Nucl Med Mol Imaging. 2021
Fiechter et al., Thromb Haemost. 2019
Gebhard et al., Sci Total Environ. 2019
Gebhard et al., PLoS One. 2018
Toma et al., Clin Res Cardiol. 2018
Stähli et al., Am J Cardiol. 2017
Stähli et al., Cardiology. 2015
Sex, Gender, and the Sympathetic Nervous System
Increasing evidence suggests that women are particularly vulnerable to the detrimental associations of mental stress and cardiovascular health. As such, women seem to perceive greater psychological stress and excessive sympathetic discharge following an acute myocardial infarction, and stress-induced cardiomyopathy, the so-called Takotsubo syndrome, mainly affects postmenopausal women. The underlying psychological and biological mechanisms accounting for the adverse response to psychological stress in women are not well understood. Besides sex differences in vascular function, variation in baseline sympathetic activity and women’s propensity towards microcirculatory abnormalities, as well as a differential activation of the limbic system and the hypothalamic–adrenocortical axis in men and women have been observed. To assess the underlying mechanisms that render the female cardiovascular system more susceptible towards mental stress, our team has conducted various preclinical and clinical studies to investigate sex and gender differences in the homeostasis and dysregulation of the sympathetic system and how these disparities translate into detrimental cardiovascular outcomes in women and men.